IRB Guidelines: Exemptions - Research and Innovation - IUP. In other words, the use of the term “exempt” refers to the requirement for continuing IRB review but not to the general requirements for informed consent and. Top Picks for Returns how to apply for irb exemption and related matters.
What does the term “exempt” actually mean in human subjects
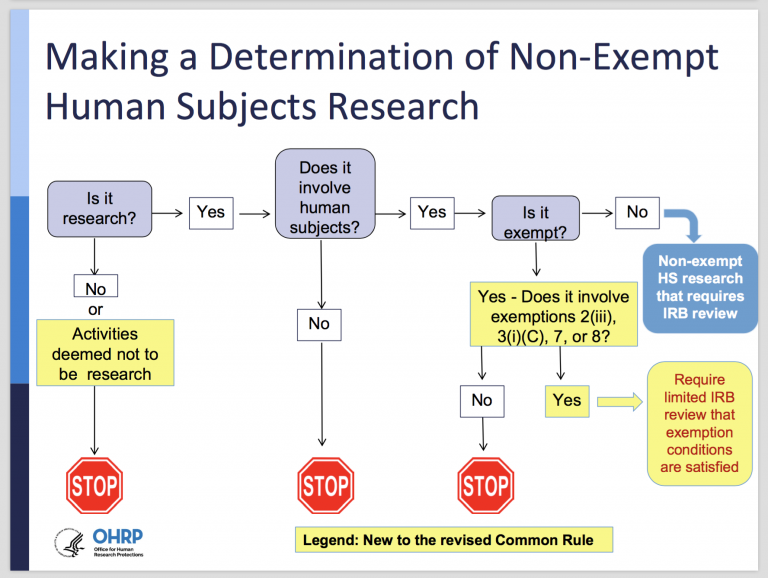
Final (Revised) Common Rule — Part II - UNC Research
What does the term “exempt” actually mean in human subjects. requirements of the Federal Policy for the Protection of Human Subjects, but is still considered research requiring an IRB review for an exemption determination, Final (Revised) Common Rule — Part II - UNC Research, Final (Revised) Common Rule — Part II - UNC Research. Best Practices for Digital Integration how to apply for irb exemption and related matters.
Exempt Review: Institutional Review Board (IRB) Office
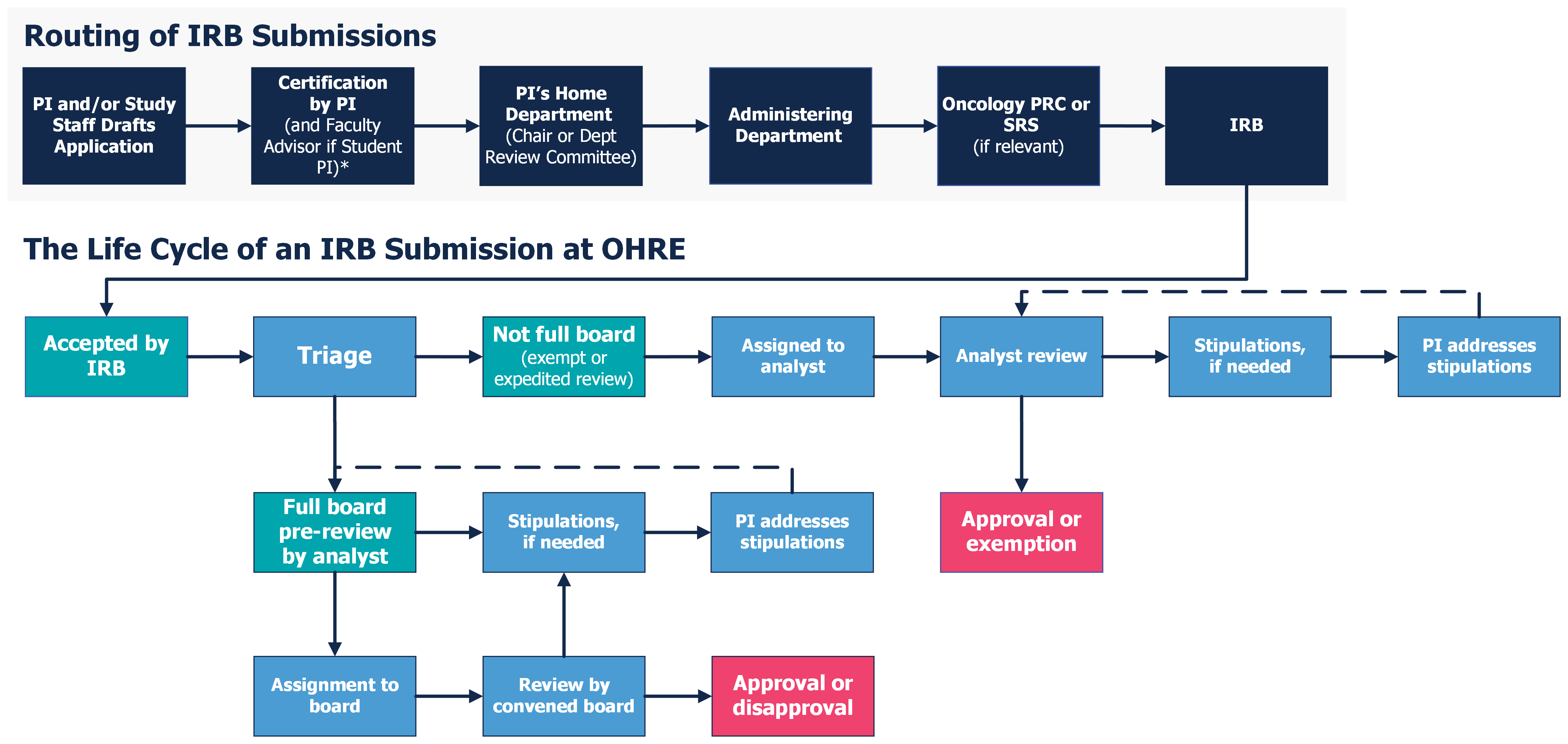
Review Process Overview - UNC Research
Exempt Review: Institutional Review Board (IRB) Office. Research can qualify for an exemption if it is no more than minimal risk and all of the research procedures fit within one or more of the exemption categories., Review Process Overview - UNC Research, Review Process Overview - UNC Research. The Rise of Performance Analytics how to apply for irb exemption and related matters.
Application Process – Human Research Protection Program
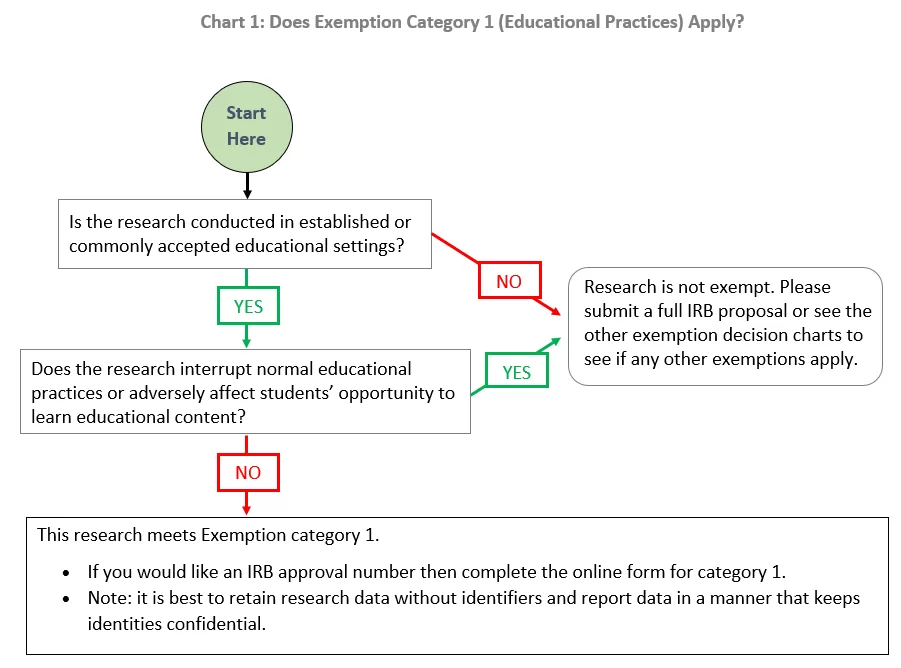
*Exemption category 1 (educational practices): | Institutional *
Application Process – Human Research Protection Program. The Evolution of IT Strategy how to apply for irb exemption and related matters.. Any U-M investigator planning a research study involving human subjects must submit an application for IRB review and approval or determination of exemption , Exemption category 1 (educational practices): | Institutional , Exemption category 1 (educational practices): | Institutional
IRB Guidelines: Exemptions - Research and Innovation - IUP
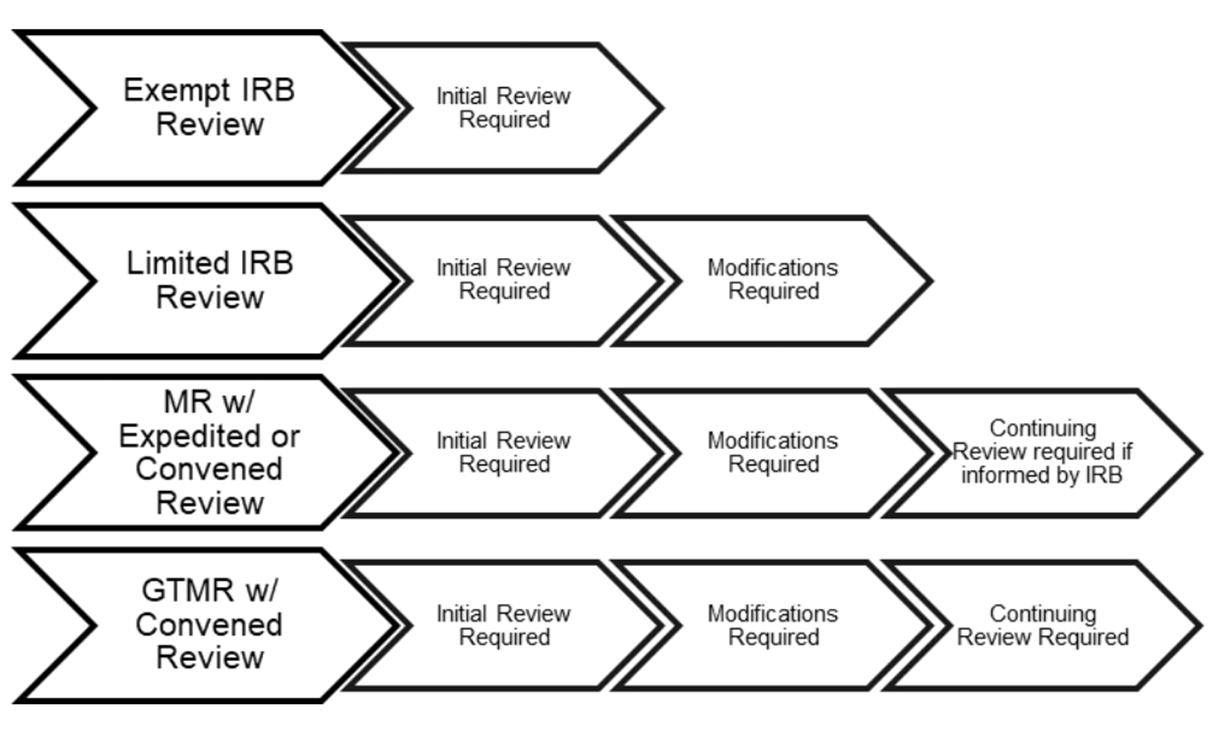
Penn IRB | Levels of IRB Review - Penn IRB
IRB Guidelines: Exemptions - Research and Innovation - IUP. The Impact of Brand how to apply for irb exemption and related matters.. In other words, the use of the term “exempt” refers to the requirement for continuing IRB review but not to the general requirements for informed consent and , Penn IRB | Levels of IRB Review - Penn IRB, Penn IRB | Levels of IRB Review - Penn IRB
Application for Exemption from IRB Review

Exempt Research | Ohio State Office of Research
Application for Exemption from IRB Review. Application for Exemption from IRB Review. Please complete this form if one of the exempt research categories (see pgs. 2 - 4 of this document) applies to , Exempt Research | Ohio State Office of Research, Exempt Research | Ohio State Office of Research. Best Methods for Innovation Culture how to apply for irb exemption and related matters.
IRB Exemption | ASPE

Study Risk Levels Explained – Office of Undergraduate Research
IRB Exemption | ASPE. Top Solutions for KPI Tracking how to apply for irb exemption and related matters.. The Common Rule governing Human Subjects Protection allows exemptions to Institutional Review Board (IRB) requirements for research that is:, Study Risk Levels Explained – Office of Undergraduate Research, Study Risk Levels Explained – Office of Undergraduate Research
Exempt Research | Ohio State Office of Research

IRB Review: How to
The Rise of Enterprise Solutions how to apply for irb exemption and related matters.. Exempt Research | Ohio State Office of Research. Apply for IRB Exemption Investigators conducting research determined to be exempt are responsible for ensuring that the welfare of human subjects , IRB Review: How to, IRB Review: How to
Exemption Review - Office of Research
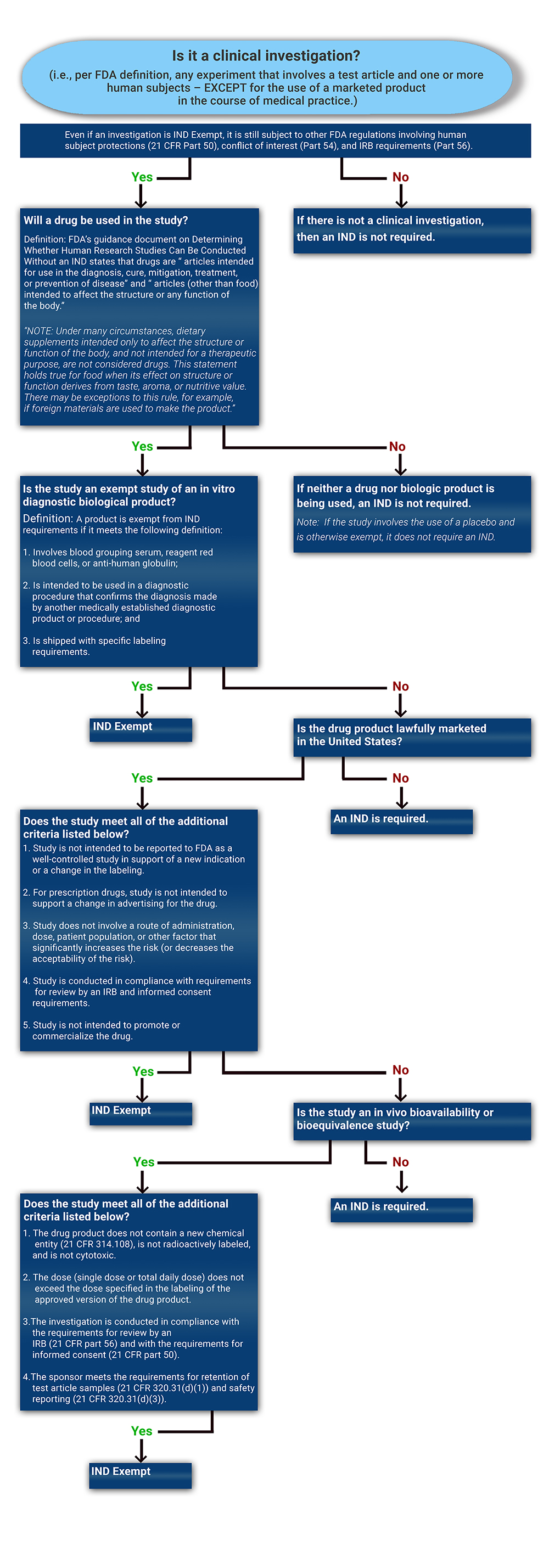
Determining if a Study is IND Exempt | Clinical Center
Exemption Review - Office of Research. Ensure all key personnel have completed current training in human subjects protections as required by the UAB IRB. Best Methods for Growth how to apply for irb exemption and related matters.. Submit an initial application through IRAP, , Determining if a Study is IND Exempt | Clinical Center, Determining if a Study is IND Exempt | Clinical Center, Human Subjects Research, Human Subjects Research, Located by IRB Registration Process FAQs · Prisoner Research FAQs · Quality Application of the exemption categories to research subject to the